Introduction to the A2O Process
In the world of modern wastewater engineering, the standard for clean water has shifted. It is no longer enough to simply remove organic solids; today’s regulations demand the removal of dissolved nutrients that threaten our ecosystems. Enter the A2O process (Anaerobic-Anoxic-Oxic).
The A2O process is a widely adopted configuration of the activated sludge system designed specifically for Biological Nutrient Removal (BNR) . Unlike traditional treatment methods that focus primarily on carbon removal, the A2O process simultaneously targets nitrogen and phosphorus —the two main culprits behind water eutrophication.
By intelligently cycling wastewater through three distinct environmental zones— Anaerobic (no oxygen, no nitrate), Anoxic (no oxygen, yes nitrate), and Oxic (aerated)—the A2O system creates a diverse ecosystem of microorganisms. These microbes work in harmony to break down organic matter, convert ammonia into harmless nitrogen gas, and biologically trap phosphorus in the sludge.
Why is the A2O Process Significant?
- Simplicity: It provides simultaneous removal of Nitrogen and Phosphorus in a single sludge system without the need for chemical additives.
- Efficiency: It utilizes the organic carbon naturally present in the wastewater to fuel the denitrification process, reducing the need for supplemental carbon sources.
- Sustainability: By reducing nutrient loads, it prevents toxic algae blooms in receiving water bodies, protecting aquatic life and human health.
Understanding Wastewater Treatment Targets
To appreciate the elegance of the A2O process, we must first understand the enemies it fights. Wastewater treatment is not just about making water look clear; it is about removing invisible chemical pollutants that disrupt nature’s balance.
While conventional treatment focuses on Carbon (measured as BOD/COD) and Solids (TSS), advanced processes like A2O are designed to tackle Nutrients .
The Three Major Pollutants
1. Organic Matter (BOD/COD)
- What it is: Biodegradable waste (food scraps, human waste).
- The Danger: If released untreated, bacteria in rivers and lakes will consume this matter aggressively. In doing so, they use up all the dissolved oxygen in the water, suffocating fish and other aquatic life.
- A2O Role: The A2O process removes organic matter primarily in the Anaerobic and Anoxic zones (using it as fuel for specific reactions) and finishes the job in the Oxic zone.
2. Nitrogen (Ammonia and Nitrates)
- What it is: Nitrogen enters wastewater primarily through urea and proteins.
- The Danger:
- Toxicity: High levels of ammonia are directly toxic to fish.
- Eutrophication: Nitrogen acts as a fertilizer for algae. When algae die and rot, they deplete oxygen (Dead Zones).
- A2O Role: The A2O process converts toxic Ammonia (NH 4 ) into Nitrate (NO 3 - ), and then strips the oxygen off to release harmless Nitrogen gas (N 2 ).
3. Phosphorus
- What it is: Found in detergents, soaps, and human waste.
- The Danger: Phosphorus is usually the “limiting nutrient” in freshwater. Even tiny additions can trigger massive, uncontrollable algae blooms that turn water green and toxic.
- A2O Role: This is the specialty of the A2O process. By stressing bacteria in the Anaerobic zone, the system primes them to absorb massive amounts of phosphorus in the Oxic zone, trapping it in the sludge so it can be removed from the water.
The A2O Process Flow: A Step-by-Step Journey
The A2O process is a continuous journey for wastewater, designed to create specific environmental conditions that favor different types of bacteria. The key to its success lies not just in the tanks themselves, but in the two critical recirculation loops that move the water and sludge between them.
1. The Anaerobic Zone (The Selector)
This is the initial contact zone where the process begins.
- Inflow: Raw influent wastewater (rich in organic “food”) is mixed with Return Activated Sludge (RAS) from the secondary clarifier.
- Environment: Strictly anaerobic. There is no dissolved oxygen (O 2 ) and no nitrates (NO 3 ).
- Key Process (P-Release): In this stressed environment, Phosphate Accumulating Organisms (PAOs) are selected. They consume Volatile Fatty Acids (VFAs) from the wastewater and, to gain the energy to do so, break down their internal polyphosphate bonds, releasing orthophosphate into the liquid.
2. The Anoxic Zone (Denitrification)
The wastewater flows from the anaerobic zone into the anoxic zone, where it is joined by a massive stream of recycled water.
- Inflow: Mixed liquor from the Anaerobic zone Internal Mixed Liquor Recycle (IMLR) from the Oxic zone.
- Environment: Anoxic. There is no free dissolved oxygen, but there is chemically bound oxygen in the form of nitrates (NO 3 ) brought in by the IMLR.
- Key Process (Denitrification): Heterotrophic bacteria use the remaining organic matter as a food source. To breathe, they strip the oxygen atoms from the nitrate molecules (NO 3 ), converting them into nitrogen gas (N 2 ), which bubbles harmlessly out of the water. This is the primary mechanism for nitrogen removal.
3. The Oxic Zone (The Aerobic Engine)
This is the largest and most active zone, where air is vigorously introduced.
- Inflow: Mixed liquor from the Anoxic zone.
- Environment: Aerobic. High levels of dissolved oxygen are maintained by diffusers or aerators.
- Key Process 1 (Nitrification): Autotrophic bacteria (like Nitrosomonas and Nitrobacter ) convert toxic ammonia (NH 4 ) into nitrates (NO 3 ).
- Key Process 2 (Luxury P-Uptake): The PAOs, now in an oxygen-rich environment, “luxury uptake” large amounts of phosphate from the water to rebuild their internal stores, removing it from the liquid phase.
- The Split: At the end of this zone, a large portion of the nitrate-rich mixed liquor is pumped back to the Anoxic zone via the IMLR , while the rest flows to the clarifier.
4. The Secondary Clarifier (Separation)
The final stage is a physical separation process.
- Inflow: Mixed liquor from the Oxic zone.
- Process: The biological flocs (sludge) settle to the bottom of the tank, leaving clear, treated water at the top.
- Outflow (Effluent): The clear supernatant flows over weirs and is discharged as treated effluent.
- Sludge Management: The settled sludge is either recycled back to the start as RAS to maintain the biological population or removed from the system as Waste Activated Sludge (WAS) to permanently remove the phosphorus and excess biomass.
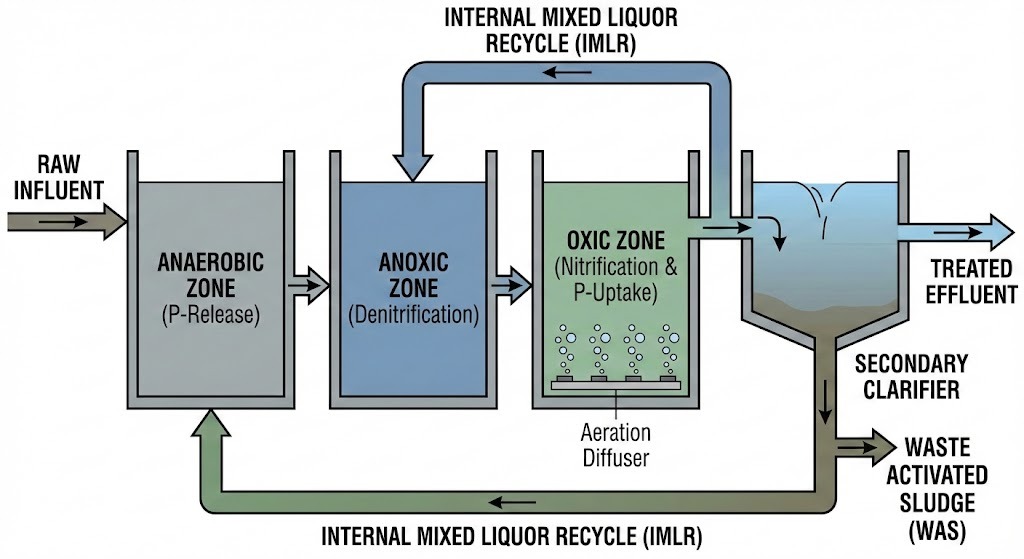
Core Stages of the A2O Process
The A2O process is a single-sludge suspended growth system. While it appears linear, its efficiency relies heavily on internal recirculation. The wastewater moves through three distinct environmental zones, each cultivating specific bacterial communities to target different pollutants.
[Image of A2O process flow diagram]
1. The Anaerobic Zone (The Selector)
This is the initial contact zone where the raw influent wastewater mixes with the Return Activated Sludge (RAS).
- The Environment: Strictly anaerobic conditions. There is no free oxygen (O 2 ) and no bound oxygen (Nitrate/Nitrite).
- The Mechanism (Phosphorus Release): In this stress-filled environment, Phosphate Accumulating Organisms (PAOs) are dominant. To survive, they consume Volatile Fatty Acids (VFAs) from the wastewater. To gain the energy required to absorb these VFAs, PAOs break down their internal polyphosphate bonds, releasing orthophosphate into the liquid.
- The Result: Ironically, phosphate concentrations increase in this stage. This “release” is a necessary precursor for the “luxury uptake” that happens later.
2. The Anoxic Zone (Denitrification)
The wastewater flows from the anaerobic zone into the anoxic zone. Here, a crucial internal recycle loop feeds nitrate-rich mixed liquor back from the end of the process (the Oxic zone).
- The Environment: Anoxic conditions. There is no free dissolved oxygen, but chemically bound oxygen is present in the form of Nitrates (NO3 - ).
- The Mechanism (Denitrification): Heterotrophic bacteria use the organic matter (BOD) remaining in the wastewater as food. To respire, they strip the oxygen molecules off the Nitrates.
- The Chemical Shift: This process converts Nitrate (NO3 - ) into Nitrogen gas (N 2 ), which bubbles harmlessly out of the water.
NO3 - → NO2 - → NO → N 2 O → N 2 - The Result: Significant removal of total nitrogen.
3. The Oxic Zone (Aerobic Treatment)
This is the final biological stage where aeration is introduced via mechanical surface aerators or diffused air systems.
- The Environment: Aerobic conditions with high Dissolved Oxygen (DO) levels (typically 2.0 mg/L or higher).
- Mechanism A (Nitrification): Autotrophic bacteria (like Nitrosomonas and Nitrobacter ) convert Ammonia (NH 4 ) into Nitrates (NO3 - ). This nitrate is then recycled back to the Anoxic zone to be removed.
- Mechanism B (Luxury Phosphorus Uptake): The PAOs, now in an oxygen-rich environment, go into overdrive. They oxidize the stored organics (absorbed in the anaerobic phase) to replenish their phosphate stores. They take up much more phosphate than they released earlier.

- The Result: Ammonia is oxidized, and the liquid phase phosphate is drastically reduced as it is trapped inside the bacteria (which will eventually be removed as sludge).
Factors Affecting A2O Process Efficiency
The A2O process is a biological balancing act. Because it relies on living microorganisms, the system is sensitive to environmental changes. To achieve optimal nutrient removal, operators must carefully monitor and control several key factors.
1. Dissolved Oxygen (DO) Control
This is the most critical parameter. The bacteria in each zone require a specific oxygen environment to function.
- Anaerobic Zone: Must be strictly anaerobic (DO ≅ 0 mg/L). Even small amounts of oxygen here will stop phosphorus release.
- Anoxic Zone: Must have low DO (DO < 0.5 mg/L) but high nitrates. If DO enters this zone (e.g., via excessive turbulence or over-aerated return sludge), bacteria will use the free oxygen instead of the nitrate oxygen, halting denitrification.
- Oxic Zone: Requires sufficient DO (2.0 - 3.0 mg/L). If levels drop too low, nitrification stops; if levels are too high, it wastes energy and sends excess oxygen back to the anoxic zone via the recycle loop.
2. Internal Recirculation Ratios
The “heartbeat” of the A2O process is its pumps.
- IMLR (Internal Mixed Liquor Recycle): This determines how much nitrate is removed. A standard ratio is 200% to 300% of the influent flow. If the ratio is too low, nitrates escape in the effluent. If it is too high, it dilutes the mixed liquor and reduces retention time.
- RAS (Return Activated Sludge): This ensures the Anaerobic zone has enough biomass. Typically set at 50% to 100% of influent flow.
3. Temperature and pH
Different bacteria have different “comfort zones.”
- Temperature: Nitrifying bacteria (Oxic zone) are very sensitive to cold. Below 12 °C , their activity drops significantly, risking high ammonia in the discharge.
- pH: Nitrification consumes alkalinity, naturally lowering the pH. If the pH drops below 6.5 , the bacteria stop working. Operators often need to add alkalinity (like lime or soda ash) to maintain a pH between 7.0 and 8.0 .
4. Carbon-to-Nutrient Ratio (C:N:P)
Bacteria need food (Carbon) to do their work.
- Denitrification requires organic carbon. If the wastewater is “weak” (low BOD), there won’t be enough food for the bacteria to break down the nitrates in the Anoxic zone.
- Phosphorus Removal relies on Volatile Fatty Acids (VFAs). If the influent lacks VFAs, phosphorus removal will be poor.
Advantages and Disadvantages of the A2O Process
While A2O is a gold standard for biological nutrient removal, it is not a “install and forget” system. It has distinct pros and cons compared to conventional activated sludge.
The Advantages (Pros)
- Simultaneous Nutrient Removal: It effectively removes BOD, Nitrogen, and Phosphorus in a single sludge system without needing separate chemical precipitation stages.
- Cost-Effective Operation: By using the nitrates (instead of air) to oxidize BOD in the anoxic zone, the process recovers oxygen, reducing the overall aeration energy demand.
- Improved Sludge Properties: The anaerobic selector zone suppresses the growth of filamentous bacteria, which often cause “sludge bulking.” This leads to better settling sludge in the clarifier.
- No Added Chemicals: It relies on biological mechanisms rather than expensive chemical coagulants (like alum or ferric chloride) for phosphorus removal.
The Disadvantages (Cons)
- Sensitivity to Influent Quality: The process depends heavily on the ratio of BOD to Nitrogen/Phosphorus in the raw sewage. If the incoming water is low in organic matter (Carbon), the removal efficiency drops drastically.
- Complexity of Operation: Balancing the two recycle loops (RAS and IMLR) requires skilled operators and precise control systems.
- Nitrate Feedback: If the internal recycle is not managed correctly, nitrates can flow back into the Anaerobic zone. Nitrates in the anaerobic zone act as a poison to the phosphorus-removal mechanism.
- Higher Initial Capital: The requirement for three separate zones, internal walls, mixers, and recycle pumps increases the upfront construction cost compared to a simple aeration tank.
Real-World Applications of A2O
The A2O process is versatile and scalable, making it a preferred choice for diverse wastewater treatment scenarios.
1. Municipal Wastewater Treatment
This is the most common application. Cities worldwide use A2O to meet strict effluent standards that forbid the discharge of nitrogen and phosphorus into rivers and lakes.
- Retrofitting: One of the greatest strengths of A2O is that many existing “plug-flow” aeration tanks can be retrofitted into A2O systems simply by installing baffles (walls) to create the three zones and adding recirculation pumps.
- Scale: It is effective for medium to large-scale plants (serving populations from 10,000 to over 1,000,000).
2. Industrial Applications
Industries that produce organic waste with high nutrient content find A2O particularly effective.
- Food & Beverage: Dairy plants, breweries, and slaughterhouses often produce wastewater with high Nitrogen and Phosphorus loads. A2O helps these facilities meet environmental discharge permits without excessive chemical costs.
- Fertilizer Plants: These facilities deal with high ammonia concentrations, making the nitrification/denitrification capabilities of A2O essential.
Maintenance and Troubleshooting
Even a perfectly designed A2O system can face operational challenges. Biological systems are dynamic; a shift in weather, influent composition, or equipment failure can disrupt the delicate balance of bacteria.
Common Operational Issues and Solutions
The table below outlines the most frequent problems operators face in A2O plants and how to fix them.
| Symptom | Probable Cause | Action / Solution |
| Poor Phosphorus Removal | Nitrates in Anaerobic Zone: If nitrates enter the first zone, bacteria will use them instead of fermenting. This stops P-release. | Check RAS: Reduce the Return Activated Sludge (RAS) rate or optimize the denitrification in the Anoxic zone to ensure no nitrates are left in the return sludge. |
| Floating Sludge (Clumping) | Denitrification in Clarifier: If sludge sits too long in the secondary clarifier, it runs out of oxygen. Bacteria start converting nitrates to nitrogen gas in the clarifier , causing sludge clumps to float to the surface. | Increase RAS Rate: Pump the sludge out of the clarifier faster to prevent it from going anoxic.
Reduce SRT: Lower the sludge age slightly. |
| High Effluent Ammonia | Loss of Nitrification: Nitrifying bacteria are sensitive. Causes include low pH, cold temperatures, or toxins. | Check DO & pH: Ensure Oxic zone DO is >2.0 mg/L and pH is >7.0.
Increase SRT: Increase the sludge age to allow slow-growing nitrifiers to recover. |
| Foaming / Scum | Filamentous Bacteria: Organisms like Nocardia or Microthrix thrive when there is high grease or low F/M (Food to Microorganism) ratio. | Skimming: Physically remove the foam.
Chlorination: Careful dosing of chlorine on the return sludge can kill filaments without killing the biomass. |
| Turbid Effluent | Dispersed Growth: Bacteria aren’t forming good “flocs” (clumps) and won’t settle. | Reduce Aeration Shearing: High turbulence can break flocs.
Check Toxicity: Look for industrial toxins entering the plant. |
Preventive Maintenance Tips
- Sensor Calibration: The A2O process relies on DO and Nitrate sensors to control pumps. Calibrate these weekly.
- Mixer Maintenance: The Anaerobic and Anoxic zones use submersible mixers to keep solids suspended without adding oxygen. If a mixer fails, solids will settle and reduce the effective tank volume.
- Pump Inspection: The internal recycle pumps (IMLR) run continuously. Regular vibration analysis and seal checks are vital to prevent sudden failure.
Frequently Asked Questions (FAQ) about the A2O Process
Q: What is the main difference between the A/O process and the A2O process?
A: The standard A/O (Anaerobic-Oxic) process is designed primarily for Phosphorus removal. It lacks the “Anoxic” zone and the internal nitrate recycle, meaning it cannot effectively remove Nitrogen. The A2O (Anaerobic-Anoxic-Oxic) adds that middle step to remove both Nitrogen and Phosphorus.
Q: Why must the Anaerobic zone be free of Nitrates?
A: If nitrates are present in the Anaerobic zone, the bacteria will use the oxygen from the nitrates to breathe instead of fermenting the wastewater. This prevents the “stress” condition needed for Phosphorus Accumulating Organisms (PAOs) to release phosphorus, effectively breaking the biological phosphorus removal process.
Q: What is the typical removal efficiency of an A2O system?
A: A well-operated A2O plant can typically achieve:
- BOD/COD: > 90%
- Total Nitrogen (TN): 60% – 80% (Limited by the internal recycle ratio)
- Total Phosphorus (TP): 70% – 90%
Q: What is MLSS and why is it important in A2O?
A: MLSS stands for Mixed Liquor Suspended Solids . It is a measure of the concentration of bacteria (biomass) in the tank. In A2O systems, MLSS is usually maintained between 3,000 mg/L and 5,000 mg/L. If it’s too low, there aren’t enough bacteria to treat the water; if it’s too high, the clarifier may get overloaded.
Q: Can the A2O process meet strict Total Nitrogen limits (e.g., < 3 mg/L)?
A: Standard A2O often struggles to hit very low nitrogen limits because it relies on a single internal recycle loop. To meet limits below 3-5 mg/L, plants often need a secondary anoxic zone (Modified Bardenpho process) or the addition of an external carbon source (like methanol) to boost denitrification.
Q: Why is my A2O plant experiencing “rising sludge” in the clarifier?
A: Rising sludge is usually caused by uncontrolled denitrification in the clarifier. If the sludge sits there too long, bacteria convert remaining nitrates into nitrogen gas bubbles, which stick to the sludge and float it to the surface. The solution is to increase the Return Activated Sludge (RAS) rate to get the sludge out of the clarifier faster.
 +86-15267462807
+86-15267462807

